Examples of test questions
Examples of test questions for Research in Biomedicine and Health
Test your knowledge!
(Answers are available in the textbook Principles of Research in Medicine, Appendix VI)
There are three types of questions, marked I, II or III in brackets at the beginning of each question.
- Type I: There are five possible answers to the question. Chose a single answer – A, B, C, D or E.
- Type II: There are for possible answers to the question – 1 to 4. If the most correct answers are: 1, 2 and 3 – mark A; 1 and 3 – mark B; 2 and 4 – mark C; only 4 – mark D; 1, 2, 3, and 4 – mark E.
- Type III: The question consists of a statement and its explanation. Both can be correct or incorrect. If both are correct, they may or may not be related. Therefore, there are five possible answers: correct, correct, related – mark A; correct, correct, not related – mark B; correct, incorrect – mark C; incorrect, correct – mark D; incorrect, incorrect – mark E.
1. (I) Scientific researcher is the person who
A. works hard in a specific field, has extensive knowledge about the field and has limited number of published papers in scientific journals.
B. works on a very wide field of investigation, but has no publications.
C. has extensive knowledge about the field, although does not work in research.
D. does not work hard, but is very intelligent.
E. is intelligent, has extensive knowledge, works hard and does not have to achieve scientific acknowledgement through publications.
2. (II) Scientific investigation is important because we believe that
1. our knowledge needs to be additionally proven;
2. research can bring novel findings;
3. research has high priority in society;
4. it is important to find correct answers to research questions.
3. (III) University professors need to be scientists as well
because:
they need to have scientific achievements for academic carrier.
4. (III) Scientific medicine does not need to argue against “complementary and alternative” medicine
because:
we do not have criteria of ultimate truth.
5. (II) Statistics is important in medicine, because
1. we can never be 100% sure about a finding;
2. samples in medical research are often very small;
3. medical tests have wide range of normal values;
4. it enables us to predict reliable prognosis for the individual patient.
6. (I) If we conclude that immune system protects from cancer on the basis of the fact that kidney transplant recipients who receive immunosuppression develop cancer more often, then we
A. concluded inductively.
B. showed no knowledge of immunology.
C. set up a new theory.
D. confronted the paradigm.
E. observed the novel finding.
7. (III) When two hypothesis are of similar value, it is better to test one that contradicts more known facts
because:
by setting the hypothesis we intend to refute the existing theory.
8. (I) All statements about evidence based medicine are true, EXCEPT
A. clinical decisions based exclusively on evidence.
B. critical application of the best existing evidence in making decision about the patient treatment.
C. incorporation of research findings in every-day clinical practice.
D. procedure of systematic searching, assessment and use of novel scientific findings as a basis for decisions in clinical practice.
E. clinical practice in which we are aware of existing evidence that support our decisions, and can assess its power.
9. (III) Government may restrict the application of certain scientific result
because:
government provides a significant financial support for scientific investigation.
10. (I) The control group in comparison with the experimental group needs to be
A. different in as many characteristics as possible.
B. different in as few characteristics as possible.
C. identical.
D. different in every other characteristics but the one tested.
E. different only in the characteristic we are testing.
11. (I) New laboratory test detects antibodies in 10 out of 50 serum samples, whereas the old test detects no antibodies in any of the serum samples. Specificity of the new test
A. is equal to sensitivity.
B. can not be calculated.
C. is 0.95.
D. is 0.8.
E. is 0.2.
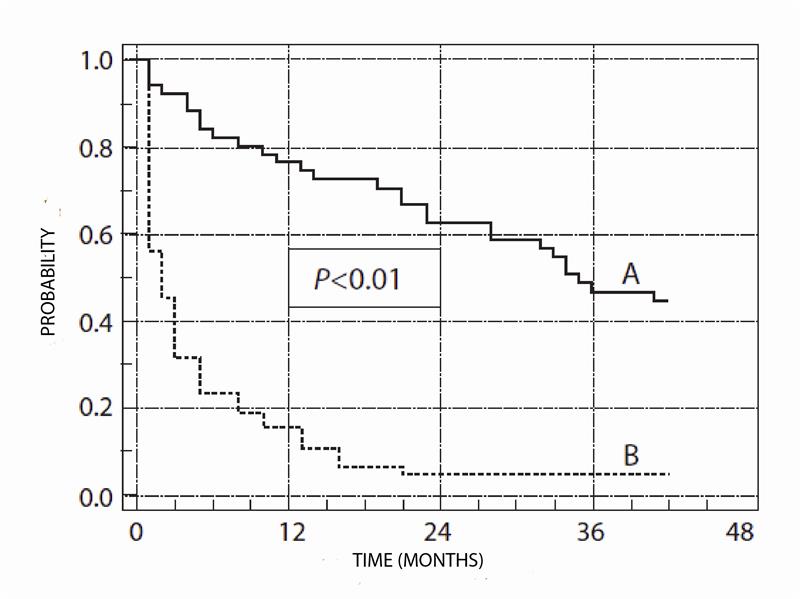 Figure 1.
Figure 1. Disease-free survival in patients with malignant skin melanoma (day 0 - surgery). A - tumor thickness <4.2 mm (n = 57 patients); B - tumor thickness ³4.2 mm (n = 63 patients).
12. (I) Analysis of disease-free survival in patients with malignant skin melanoma presented in Figure 1 tells us that
A. disease-free survival is similar in both groups, since there is no statistically significant difference.
B. disease-free survival is much greater in group B.
C. five-year disease-free survival is better in group A than in group B.
D. tumor thickness of <4.2 mm is more favorable to achieve better disease-free survival.
E. disease-free survival is better if we do not assess it in relation to tumor thickness.
13. (I) What can be concluded from Figure 1?
A. Probability of disease-free survival in group A is 30% one year after surgery.
B. Probability of disease-free survival in group A is 80% one year after surgery.
C. Probability of disease-free survival in group A decreased from 100% to 5% in a three-year follow up.
D. Disease-free survival in group A is more that 50% for more than two years.
E. Disease-free survival in group B is more that 50% for more than a year.
14. (I) In Figure 1, disease-free survival of patients with melanoma are presented as
A. scatter diagram.
B. histogram.
C. algorithm.
D. linear graph.
E. none of the above.
15. (II) Laboratory test I detect pregnancy in 40 out of 44 pregnant women, whereas laboratory test II detect pregnancy in 32 out of 38 pregnant women. Therefore, one can conclude that
1. percent of false negative results is lower for test I than II;
2. percent of false positive results can not be calculated for either test;
3. sensitivity of test I is greater than of test II;
4. specificity can not be calculated for either test.
16. (II) Two groups of laboratory animals have different glucose concentrations in blood. Before we apply a statistical test to prove the difference between groups, we need to
1. set the statistical hypothesis;
2. randomly distribute animals into two groups;
3. set the alpha-value;
4. calculate mean and median of the obtained data.
17. (II) To investigate the effect of a new drug by randomized controlled trial, it is necessary to
1. previously use the drug as a routine therapy in clinical practice;
2. randomly distribute participants to research groups;
3. analyze the patients’ survival;
4. assembly appropriate control group.
18. (I) For diagnostic procedure, choose the best possible test (described by the following values of sensitivity and specificity)
A. sensitivity < 0.01; specificity < 0.05.
B. sensitivity = 0.05; specificity = 0.05.
C. sensitivity = 0.95; specificity = 0.05.
D. sensitivity = 0.95; specificity = 0.92.
E. sensitivity = 0.85; specificity = 0.01.
19. (III) Sensitivity of the laboratory procedure is the ability to recognize the tested characteristic
because:
sensitivity is the ratio of false positive results and total number of tests.
20. (I) All statements about prospective studies are correct, EXCEPT
A. research plan needs to specify the time-period for the application of experimental intervention.
B. experimental results do not exist at the beginning of the research.
C. researcher should search for the existing knowledge about the research problem in advance.
D. they usually cost less than retrospective studies.
E. researcher can control experimental conditions during data collection.
21. (II) To deserve the authorship on a research article one would
1. prepare the experimental plan;
2. critically review the research hypothesis;
3. participate in data collection and analysis;
4. participate in statistical analysis of data.
22. (II) Correct statement(s) about “Material and methods” section is(are)
1. novel structure of the clinical study needs to be described in detail;
2. ethical principles for the sampling method need to be carefully described;
3. for standard laboratory test it is enough to give the name of the method and citation;
4. different journals have the same structure of “Material and methods” section.
23. (III) It is desirable to present the same results both in tables and graphs
because:
combination of tables and figures makes research article more interesting.
24. (I) Good scientific prose is characterized by all statements, EXCEPT
A. clear writing.
B. simple and short sentences.
C. accurate writing.
D. detailed writing and long text.
E. logical structure of the text.
25. (II) Correct statement(s) regarding the editors decision about the acceptance of research article for publication is(are)
1. acceptance without revision is rare;
2. all reviewers’ requests need to be accepted;
3. for resubmission, corrected manuscript needs to be accompanied by the letter of explanation;
4. manuscript rejected by one journal can not be published in another journal.
26. (II) Important element(s) in scoring a scientific project is(are)
1. importance of the project for scientific priorities of the institution;
2. importance of the project for regional and national priorities;
3. researchers’ competence;
4. laboratory resources.
27. (I) Disadvantages of a randomized controlled trial are all listed, EXCEPT
A. it allows comparison of only two groups.
B. it is difficult to obtain sample large enough for the study.
C. it is often financially supported by companies, which dictate study content and execution.
D. many of them are never completed.
E. many of them do not last long enough.
28. (III) Randomized controlled trial is the most appropriate type of study design to investigate the effect of certain drug
because:
randomized controlled trials are used for meta-analysis.
29. (II) To investigate the association between possible risk factor and disease, it is appropriate to chose
1. cohort study;
2. randomized controlled trial;
3. case-control study;
4. cross-section study.
30. (I) Advantages of randomized controlled trial are all listed, EXCEPT
A. investigates the causal relationship.
B. it is prospective in nature.
C. it is easy to organize.
D. it allows meta-analysis.
E. uses the hypothetic-deductive principle.
31. (I) The important characteristic of
Science Citation Index is
A. index of subject headings.
B. author index.
C. permuted index.
D. citation index.
E. corporative index.
32. (I) To search the library to find the literature about the specific topic, you will use
A. author catalogue.
B. catalogue of audio-visual material.
C. MeSH (Medical Subject Headings) catalogue.
D. catalogue of periodic publications.
E. any of the above.
33. (I) After formulating the relevant clinical question, which sources of evidence you will choose first?
A. Medline.
B. Science Citation Index.
C. The Cochrane database of systematic reviews.
D. Current Contents.
E. EMBASE.
34. (III) Impact factor reflects the scientific impact of the journal
because:
impact factor of the journal measures the number of citations per paper.
35. (I) 95% confidence interval
A. should be given only when difference between groups is statistically significant.
B. tells us that the mean of the population is within the given range with 95% probability.
C. does not tell us anything about the significance of the difference between groups.
D. is the most reliable measure of the mean value.
E. is never used with the mean value.
36. (II) As a strategy to find particular scientific information, researcher needs to
1. precisely define the topic of the search;
2. choose appropriate database for the search;
3. choose appropriate subject headings for the search;
4. set the time-period for the search source.
37. (II) Primary publication(s) is(are)
1. original research article published in a scientific journal;
2. bibliography;
3. doctoral thesis;
4. abstracts book.
38. (II) MeSH (
Medical Subject Headings) is
1. list of currently recommended subject headings used to search for medical literature by content;
2. alphabet list of subject headings that is used for searching some databases;
3. hierarchical thesaurus that organizes our knowledge in a hierarchical list of related headings;
4. classification protocol that is used to sort library material.
39. (III) Researchers need to publish obtained results in international scientific journals
because:
in that way, authors make their findings visible to the scientific community.
40. (III) When writing the research article, researcher should cite literature used for the study
because:
citation of the used literature is a common way to acknowledge research work of other authors.
Print page
